Conventionally the parameter Z' is used to denote the number of molecules in the asymmetric unit. Of course, Z' is therefore crucially dependent on the somewhat subjective definition of what constitutes the 'formula unit'. Strictly it is defined as the number of formula units in the unit cell divided by the number of independent general positions. The complexity of the asymmetric unit derives from the fact that the molecules within the asymmetric unit are free to associate themselves in three-dimensional space, unlike the asymmetric units themselves which are compelled to tessellate via particular symmetry operators (to form one of the 230 space groups).
In the past, refinement and solution of high Z' structures was often problematic due to large unit cells containing a sizeable number of non-hydrogen atoms, however since the advent of CCD and area detector systems as well as enhanced computer capability these problems are now much reduced.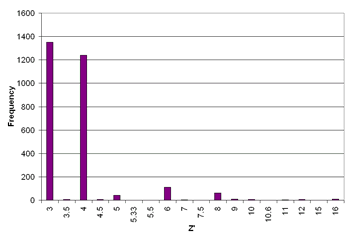
Around 8.8%1 of structures in the Cambridge Structural Database crystallise with Z' > 1 with values ranging from 1 1/12 to 32. Even numbers of Z' are more common than odd numbers and the number of structures with Z' > 4 is negligible (see graph of 3 ≤ Z' ≤ 16, left). Certain classes of compound, particularly chiral molecules, such as nucleosides, nucleotides, steroids and other natural products2 show a greater tendency to form high Z' structures. Strong intermolecular interactions such as the highly directional O-H···O interaction have been implicated in the formation of high Z' structures, although there are also many high Z' structures known which arise from only weak interactions (e.g. the rhenium(I) complex [Re(MeCN)Cl2(NO)(PMe3)2] WODCOH, Z' = 11 which is held together predominantly by C-H···O and C-H···Cl hydrogen bonds).
Broadly we believe that high Z' structures arise from frustration between two or more competing interactions (e.g. optimisation of hydrogen bonds vs. optimal shape packing) either during the nucleation process or as a dominant contribution to the bulk structure. We have explicitly probed this frustration concept through a database and experimental study combining centrosymmetric synthons and resolved chiral compounds which demonstrates an overwhelming tendency towards Z' > 1.3
The observation of Z' > 1 may be regarded as a special case of cocrystallisation where the two components are the same as one another. Thus research on high Z' structures is closely related to cocrystals, solvates, hydrates etc. An additional parameter, Z'', has been used to denote the total number of molecules (of whatever type) present in the asymmetric unit.4,5 Reported high Z' structures may also be approximations to modulated structures that might be better handled by superspace refinement methods.6
The nucleation process may also be a good source of information on high Z' structures as it has been postulated that many high Z' structures are "fossil relics" of the fastest growing crystal nucleus7, i.e. a metastable polymorph. Techniques such as Differential Scanning Calorimetry (DSC) or variable temperature X-ray crystallography can be used to probe high Z' structures for polymorphic behaviour.
For a more detailed description of our research into the Z' phenomenon please click here.
References
- K. M. Anderson, A. E. Goeta, K. S. B. Hancock and J. W. Steed, Chem. Commun., 2006, 2138
- T. Steiner, Acta Cryst., Sect. B, 2000, 56, 673.
- K. M. Anderson, K. Afarinkia, H-W. Yu, A. E. Goeta and J. W. Steed, Cryst. Growth Des., 6, 2109.
- W. Clegg and G. S. Nichol, Cryst. Growth Des., 2006, 6, 451.
- B. P. van Eijck and J. Kroon, Acta Crystallogr., Sect. B, 2000, 56, 535
- (a) M. Meyer, W. A. Paciorek, K. J. Schenk, G. Chapuis, and W. Depmeier, Acta Crystallogr, Sect. B, 1994, 50, 333. (b) W. Paciorek, V. B. Gaillard, K. Schenk, and G. Chapuis, Acta Crystallogr., Sect. A, 1996,52, 349
- (a) J. W. Steed, CrystEngComm, 2003, 169. (b) D. Das, R. Banerjee, R. Mondal, J. A. K. Howard, R. Boese, and G. R. Desiraju, Chem. Commun., 2006, 555.